
SL Paper 2
Ethane-1,2-diol, HOCH2CH2OH, has a wide variety of uses including the removal of ice from aircraft and heat transfer in a solar cell.
Ethane-1,2-diol can be formed according to the following reaction.
2CO (g) + 3H2 (g) HOCH2CH2OH (g)
(i) Deduce the equilibrium constant expression, Kc, for this reaction.
(ii) State how increasing the pressure of the reaction mixture at constant temperature will affect the position of equilibrium and the value of Kc.
Position of equilibrium:
Kc:
(iii) Calculate the enthalpy change, ΔHθ, in kJ, for this reaction using section 11 of the data booklet. The bond enthalpy of the carbon–oxygen bond in CO (g) is 1077kJmol-1.
(iv) The enthalpy change, ΔHθ, for the following similar reaction is –233.8 kJ.
2CO(g) + 3H2(g) HOCH2CH2OH (l)
Deduce why this value differs from your answer to (a)(iii).
Determine the average oxidation state of carbon in ethene and in ethane-1,2-diol.
Ethene:
Ethane-1,2-diol:
Explain why the boiling point of ethane-1,2-diol is significantly greater than that of ethene.
Ethane-1,2-diol can be oxidized first to ethanedioic acid, (COOH)2, and then to carbon dioxide and water. Suggest the reagents to oxidize ethane-1,2-diol.
Many reactions are in a state of equilibrium.
The equations for two acid-base reactions are given below.
HCO3– (aq) + H2O (l) H2CO3 (aq) + OH– (aq)
HCO3– (aq) + H2O (l) CO32– (aq) + H3O+ (aq)
The following reaction was allowed to reach equilibrium at 761 K.
H2 (g) + I2 (g) 2HI (g) ΔHθ < 0
Outline the effect, if any, of each of the following changes on the position of equilibrium, giving a reason in each case.
Identify two different amphiprotic species in the above reactions.
State what is meant by the term conjugate base.
State the conjugate base of the hydroxide ion, OH–.
A student working in the laboratory classified HNO3, H2SO4, H3PO4 and HClO4 as acids based on their pH. He hypothesized that “all acids contain oxygen and hydrogen”.
Evaluate his hypothesis.
White phosphorus is an allotrope of phosphorus and exists as P4.
An equilibrium exists between PCl3 and PCl5.
PCl3 (g) + Cl2 (g) PCl5 (g)
Sketch the Lewis (electron dot) structure of the P4 molecule, containing only single bonds.
Write an equation for the reaction of white phosphorus (P4) with chlorine gas to form phosphorus trichloride (PCl3).
Deduce the electron domain and molecular geometry using VSEPR theory, and estimate the Cl–P–Cl bond angle in PCl3.
Explain the polarity of PCl3.
Calculate the standard enthalpy change (ΔH⦵) for the forward reaction in kJ mol−1.
ΔH⦵f PCl3 (g) = −306.4 kJ mol−1
ΔH⦵f PCl5 (g) = −398.9 kJ mol−1
State the equilibrium constant expression, Kc, for this reaction.
State, with a reason, the effect of an increase in temperature on the position of this equilibrium.
A mixture of 1.00 mol SO2(g), 2.00 mol O2(g) and 1.00 mol SO3(g) is placed in a 1.00 dm3 container and allowed to reach equilibrium.
2SO2(g) + O2(g) 2SO3(g)
Distinguish between the terms reaction quotient, Q, and equilibrium constant, Kc.
The equilibrium constant, Kc, is 0.282 at temperature T.
Deduce, showing your work, the direction of the initial reaction.
PCl5(g) and Cl2(g) were placed in a sealed flask and allowed to reach equilibrium at 200 °C. The enthalpy change, ΔH, for the decomposition of PCl5(g) is positive.
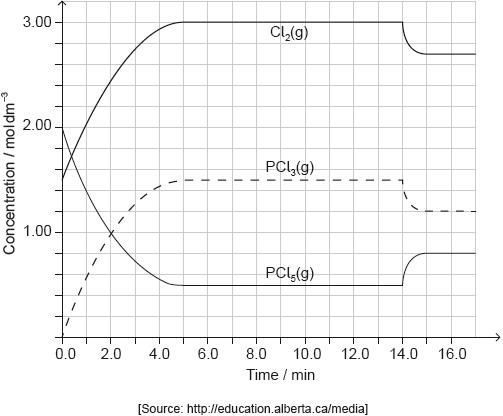
Deduce the equilibrium constant expression, Kc, for the decomposition of PCl5(g).
Deduce, giving a reason, the factor responsible for establishing the new equilibrium after 14 minutes.
Deduce the Lewis (electron dot) structure and molecular geometry of PCl3.
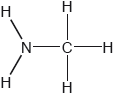
Two hydrides of nitrogen are ammonia and hydrazine, N2H4. One derivative of ammonia is methanamine whose molecular structure is shown below.
Hydrazine is used to remove oxygen from water used to generate steam or hot water.
N2H4(aq) + O2(aq) → N2(g) + 2H2O(l)
The concentration of dissolved oxygen in a sample of water is 8.0 × 10−3 gdm−3.
Estimate the H−N−H bond angle in methanamine using VSEPR theory.
Ammonia reacts reversibly with water.
NH3(g) + H2O(l) NH4+(aq) + OH−(aq)
Explain the effect of adding H+(aq) ions on the position of the equilibrium.
Hydrazine reacts with water in a similar way to ammonia. Deduce an equation for the reaction of hydrazine with water.
Outline, using an ionic equation, what is observed when magnesium powder is added to a solution of ammonium chloride.
Hydrazine has been used as a rocket fuel. The propulsion reaction occurs in several stages but the overall reaction is:
N2H4(l) → N2(g) + 2H2(g)
Suggest why this fuel is suitable for use at high altitudes.
Determine the enthalpy change of reaction, ΔH, in kJ, when 1.00 mol of gaseous hydrazine decomposes to its elements. Use bond enthalpy values in section 11 of the data booklet.
N2H4(g) → N2(g) + 2H2(g)
The standard enthalpy of formation of N2H4(l) is +50.6 kJmol−1. Calculate the enthalpy of vaporization, ΔHvap, of hydrazine in kJmol−1.
N2H4(l) → N2H4(g)
(If you did not get an answer to (f), use −85 kJ but this is not the correct answer.)
Calculate, showing your working, the mass of hydrazine needed to remove all the dissolved oxygen from 1000 dm3 of the sample.
Calculate the volume, in dm3, of nitrogen formed under SATP conditions. (The volume of 1 mol of gas = 24.8 dm3 at SATP.)
Urea, (H2N)2CO, is excreted by mammals and can be used as a fertilizer.
Calculate the percentage by mass of nitrogen in urea to two decimal places using section 6 of the data booklet.
Suggest how the percentage of nitrogen affects the cost of transport of fertilizers giving a reason.
The structural formula of urea is shown.

Predict the electron domain and molecular geometries at the nitrogen and carbon atoms, applying the VSEPR theory.

Urea can be made by reacting potassium cyanate, KNCO, with ammonium chloride, NH4Cl.
KNCO(aq) + NH4Cl(aq) → (H2N)2CO(aq) + KCl(aq)
Determine the maximum mass of urea that could be formed from 50.0 cm3 of 0.100 mol dm−3 potassium cyanate solution.
Urea can also be made by the direct combination of ammonia and carbon dioxide gases.
2NH3(g) + CO2(g) (H2N)2CO(g) + H2O(g) ΔH < 0
Predict, with a reason, the effect on the equilibrium constant, Kc, when the temperature is increased.
Suggest one reason why urea is a solid and ammonia a gas at room temperature.
Sketch two different hydrogen bonding interactions between ammonia and water.
The combustion of urea produces water, carbon dioxide and nitrogen.
Formulate a balanced equation for the reaction.
The mass spectrum of urea is shown below.
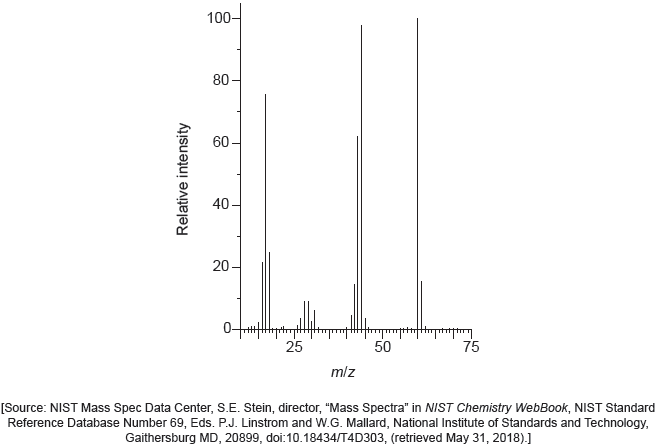
Identify the species responsible for the peaks at m/z = 60 and 44.
The IR spectrum of urea is shown below.
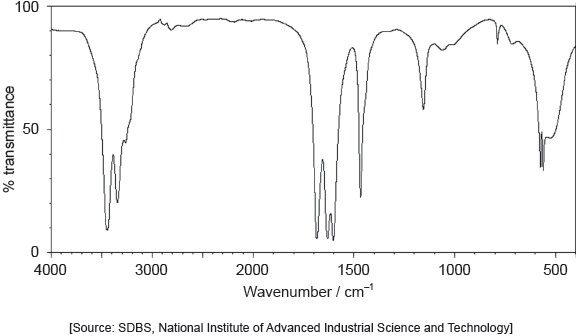
Identify the bonds causing the absorptions at 3450 cm−1 and 1700 cm−1 using section 26 of the data booklet.
Predict the number of signals in the 1H NMR spectrum of urea.
Compound A is in equilibrium with compound B.
Predict the electron domain and molecular geometries around the oxygen atom of molecule A using VSEPR.
The IR spectrum of one of the compounds is shown:
COBLENTZ SOCIETY. Collection © 2018 copyright by the U.S. Secretary of Commerce on behalf of the United States of America. All rights reserved.
Deduce, giving a reason, the compound producing this spectrum.
Compound A and B are isomers. Draw two other structural isomers with the formula .
The equilibrium constant, , for the conversion of A to B is in water at .
Deduce, giving a reason, which compound, A or B, is present in greater concentration when equilibrium is reached.
The properties of elements can be predicted from their position in the periodic table.
Explain why Si has a smaller atomic radius than Al.
Explain the decrease in radius from Na to Na+.
State the condensed electron configurations for Cr and Cr3+.
Describe metallic bonding and how it contributes to electrical conductivity.
Deduce the Lewis (electron dot) structure and molecular geometry of sulfur dichloride, SCl2.
Suggest, giving reasons, the relative volatilities of SCl2 and H2O.
Consider the following equilibrium reaction:
2SO2 (g) + O2 (g) 2SO3 (g)
State and explain how the equilibrium would be affected by increasing the volume of the reaction container at a constant temperature.
Hydrogen peroxide can react with methane and oxygen to form methanol. This reaction can occur below 50°C if a gold nanoparticle catalyst is used.
Methanol is usually manufactured from methane in a two-stage process.
CH4 (g) + H2O (g) CO (g) + 3H2 (g)
CO (g) + 2H2 (g) CH3OH (l)
Consider the first stage of the reaction.
CH4 (g) + H2O (g) CO (g) + 3H2 (g)
The diagram shows the Maxwell-Boltzmann curve for the uncatalyzed reaction.
Draw a distribution curve at a lower temperature (T2) and show on the diagram how the addition of a catalyst enables the reaction to take place more rapidly than at T1.
The hydrogen peroxide could cause further oxidation of the methanol. Suggest a possible oxidation product.
Determine the overall equation for the production of methanol.
8.00 g of methane is completely converted to methanol. Calculate, to three significant figures, the final volume of hydrogen at STP, in dm3. Use sections 2 and 6 of the data booklet.
Determine the enthalpy change, ΔH, in kJ. Use section 11 of the data booklet.
Bond enthalpy of CO = 1077 kJ mol−1.
State the expression for Kc for this stage of the reaction.
State and explain the effect of increasing temperature on the value of Kc.